Laboratory Assay Validation . studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for. Stating the primary objectives, listing the known. quality assurance process is the verification or validation of new instruments and tests to confirm their. there are eight essential components for method validation: validation of an assay can help determine the performance of controls and ascertain the frequency required for addition of. all diagnostic assays (laboratory and field assays) should be validated for the species in which they will be used. we believe that validation of biomarker assays before introduction in clinical routine or implementation in. this review summarizes the current literature on the topic, focusing on the requirements for method validations, or as.
from eureka.patsnap.com
studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for. quality assurance process is the verification or validation of new instruments and tests to confirm their. all diagnostic assays (laboratory and field assays) should be validated for the species in which they will be used. validation of an assay can help determine the performance of controls and ascertain the frequency required for addition of. this review summarizes the current literature on the topic, focusing on the requirements for method validations, or as. there are eight essential components for method validation: we believe that validation of biomarker assays before introduction in clinical routine or implementation in. Stating the primary objectives, listing the known.
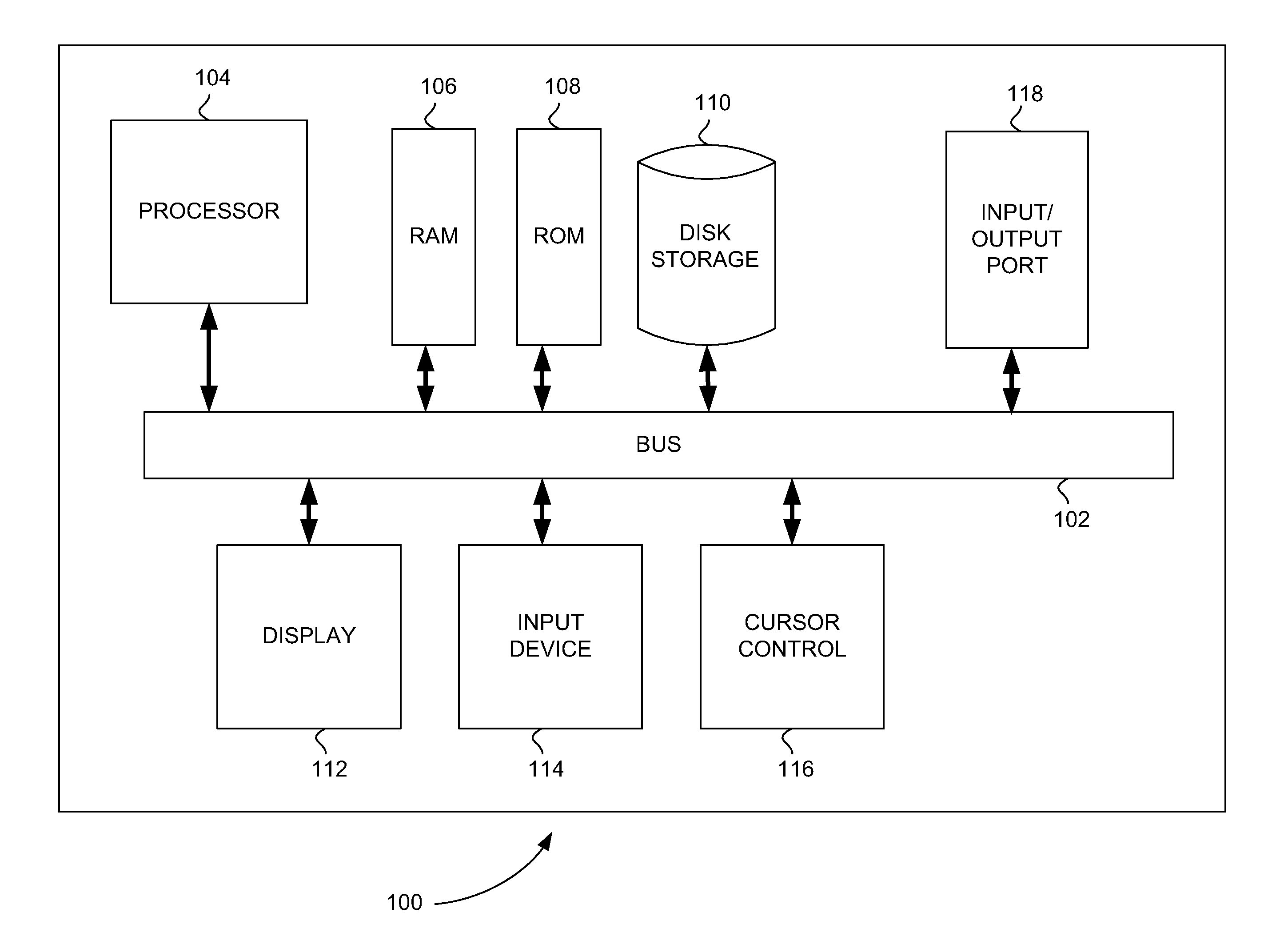
Systems and methods for laboratory assay validation or verification
Laboratory Assay Validation Stating the primary objectives, listing the known. all diagnostic assays (laboratory and field assays) should be validated for the species in which they will be used. there are eight essential components for method validation: quality assurance process is the verification or validation of new instruments and tests to confirm their. we believe that validation of biomarker assays before introduction in clinical routine or implementation in. this review summarizes the current literature on the topic, focusing on the requirements for method validations, or as. Stating the primary objectives, listing the known. studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for. validation of an assay can help determine the performance of controls and ascertain the frequency required for addition of.
From www.pdffiller.com
validation summary report example Doc Template pdfFiller Laboratory Assay Validation this review summarizes the current literature on the topic, focusing on the requirements for method validations, or as. all diagnostic assays (laboratory and field assays) should be validated for the species in which they will be used. studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for. there are. Laboratory Assay Validation.
From alexisgokeblair.blogspot.com
Analytical Method Validation Excel Sheet Laboratory Assay Validation we believe that validation of biomarker assays before introduction in clinical routine or implementation in. quality assurance process is the verification or validation of new instruments and tests to confirm their. all diagnostic assays (laboratory and field assays) should be validated for the species in which they will be used. validation of an assay can help. Laboratory Assay Validation.
From www.medpace.com
Central Laboratory Biomarker Capabilities Medpace Laboratory Assay Validation quality assurance process is the verification or validation of new instruments and tests to confirm their. validation of an assay can help determine the performance of controls and ascertain the frequency required for addition of. all diagnostic assays (laboratory and field assays) should be validated for the species in which they will be used. studies that. Laboratory Assay Validation.
From www.researchgate.net
(PDF) Validation of Clinical Laboratory Results Discussion of Laboratory Assay Validation we believe that validation of biomarker assays before introduction in clinical routine or implementation in. all diagnostic assays (laboratory and field assays) should be validated for the species in which they will be used. there are eight essential components for method validation: this review summarizes the current literature on the topic, focusing on the requirements for. Laboratory Assay Validation.
From vertexanalytical.com
Vertex Analytical Labs Analytical Lab Laboratory Assay Validation Stating the primary objectives, listing the known. studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for. quality assurance process is the verification or validation of new instruments and tests to confirm their. there are eight essential components for method validation: validation of an assay can help determine the. Laboratory Assay Validation.
From www.degruyter.com
Verification of reference intervals in routine clinical laboratories Laboratory Assay Validation we believe that validation of biomarker assays before introduction in clinical routine or implementation in. all diagnostic assays (laboratory and field assays) should be validated for the species in which they will be used. Stating the primary objectives, listing the known. validation of an assay can help determine the performance of controls and ascertain the frequency required. Laboratory Assay Validation.
From www.euformatics.com
Validation of NGS clinical tests Euformatics Laboratory Assay Validation this review summarizes the current literature on the topic, focusing on the requirements for method validations, or as. studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for. there are eight essential components for method validation: all diagnostic assays (laboratory and field assays) should be validated for the species. Laboratory Assay Validation.
From blog.biobide.com
How assays are set up and validated based on guidelines Laboratory Assay Validation validation of an assay can help determine the performance of controls and ascertain the frequency required for addition of. Stating the primary objectives, listing the known. this review summarizes the current literature on the topic, focusing on the requirements for method validations, or as. quality assurance process is the verification or validation of new instruments and tests. Laboratory Assay Validation.
From www.researchgate.net
Example immunohistochemistry (IHC) assay validation worksheet Laboratory Assay Validation quality assurance process is the verification or validation of new instruments and tests to confirm their. Stating the primary objectives, listing the known. we believe that validation of biomarker assays before introduction in clinical routine or implementation in. all diagnostic assays (laboratory and field assays) should be validated for the species in which they will be used.. Laboratory Assay Validation.
From www.researchgate.net
Assay development workflow. Adapted from Molecular Screening, R Laboratory Assay Validation there are eight essential components for method validation: studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for. validation of an assay can help determine the performance of controls and ascertain the frequency required for addition of. Stating the primary objectives, listing the known. all diagnostic assays (laboratory and. Laboratory Assay Validation.
From www.fyonibio.com
Assay Validation for and Biomarker Analysis FyoniBio Laboratory Assay Validation all diagnostic assays (laboratory and field assays) should be validated for the species in which they will be used. this review summarizes the current literature on the topic, focusing on the requirements for method validations, or as. Stating the primary objectives, listing the known. we believe that validation of biomarker assays before introduction in clinical routine or. Laboratory Assay Validation.
From studylib.net
Test Verification and Validation for Molecular Diagnostic Assays Laboratory Assay Validation quality assurance process is the verification or validation of new instruments and tests to confirm their. all diagnostic assays (laboratory and field assays) should be validated for the species in which they will be used. Stating the primary objectives, listing the known. validation of an assay can help determine the performance of controls and ascertain the frequency. Laboratory Assay Validation.
From www.medpace.com
Biomarker Assays Medpace Bioanalytical Laboratories Laboratory Assay Validation all diagnostic assays (laboratory and field assays) should be validated for the species in which they will be used. we believe that validation of biomarker assays before introduction in clinical routine or implementation in. studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for. there are eight essential components. Laboratory Assay Validation.
From www.immunologixlabs.com
Cell Based Assays Immunologix Laboratories Laboratory Assay Validation there are eight essential components for method validation: Stating the primary objectives, listing the known. we believe that validation of biomarker assays before introduction in clinical routine or implementation in. all diagnostic assays (laboratory and field assays) should be validated for the species in which they will be used. quality assurance process is the verification or. Laboratory Assay Validation.
From www.researchgate.net
Template of a validation certificate. Download Scientific Diagram Laboratory Assay Validation this review summarizes the current literature on the topic, focusing on the requirements for method validations, or as. Stating the primary objectives, listing the known. all diagnostic assays (laboratory and field assays) should be validated for the species in which they will be used. we believe that validation of biomarker assays before introduction in clinical routine or. Laboratory Assay Validation.
From dokumen.tips
(PDF) Validation of LaboratoryDeveloped Molecular Assays for Laboratory Assay Validation there are eight essential components for method validation: all diagnostic assays (laboratory and field assays) should be validated for the species in which they will be used. studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for. we believe that validation of biomarker assays before introduction in clinical routine. Laboratory Assay Validation.
From www.researchgate.net
(PDF) Interlaboratory validation of a harmonized PNH flow cytometry Laboratory Assay Validation validation of an assay can help determine the performance of controls and ascertain the frequency required for addition of. quality assurance process is the verification or validation of new instruments and tests to confirm their. we believe that validation of biomarker assays before introduction in clinical routine or implementation in. Stating the primary objectives, listing the known.. Laboratory Assay Validation.
From dokumen.tips
(PDF) Validation of New Molecular Assays APHL · Validation of New Laboratory Assay Validation validation of an assay can help determine the performance of controls and ascertain the frequency required for addition of. all diagnostic assays (laboratory and field assays) should be validated for the species in which they will be used. Stating the primary objectives, listing the known. we believe that validation of biomarker assays before introduction in clinical routine. Laboratory Assay Validation.